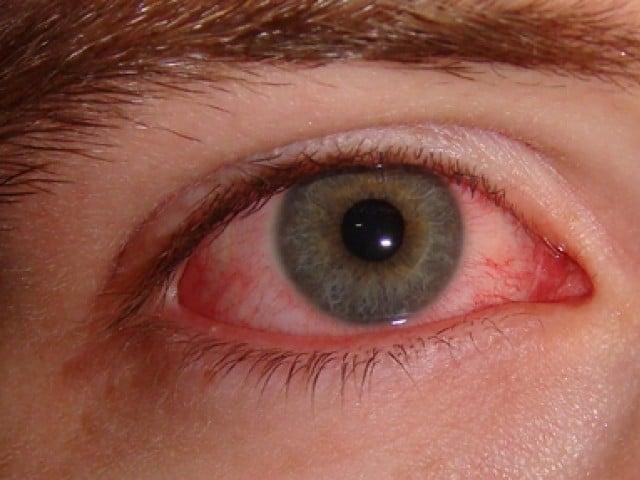
LAHORE, Sep 26: The Punjab government said on Monday it was investigating two local distributors of Swiss pharmaceutical company Roche’s Avastin cancer drug after 12 diabetic patients injected with the drug went blind.
The Drug Regulatory Authority of Pakistan (DRAP) said the health authorities in Punjab had launched the investigation into local use of the drug Avastin, which is licensed for use in Pakistan. It admitted that the use of Avastin injection caused vision loss among some patients.
“Incidents of loss of vision in diabetic patients have been reported following treatment with Altered/Dispensed/Diluted Avastin injection,” DRAP said, adding that the importer had been instructed to recall the suspected batches of Avastin 100mg injection.
Punjab Caretaker Health Minister Javed Akram said that the police were questioning two men they believed to be the drug’s distributors in the province. “A high level committee has been constituted to probe the issue. A case has been registered against the distributor and his aide,” Akram said.
DRAP said that the drug had been created illegally in the country. “The sale/distribution of registered Avastin injection has been put on halt till verification of its quality through sampling and laboratory testing to safeguard public health,” it said in a statement on its website.
On its website, Roche said Avastin was approved in more than 130 countries, including the United States, to treat several types of cancer. “In Pakistan, the vision loss from Avastin has been identified by the authorities as a case of contamination by a third party supplier,” it added.
DRAP statement said that in the cases concerning Avastin had been used off-label, meaning outside its approved use, to treat diabetes-related eye conditions. “Roche strongly condemns this criminal act of counterfeiting and is doing everything in its power to cooperate with the authorities to protect patients from counterfeits,” Roche said in a statement to Reuters.
Cancer drug Avastin, when used at much lower doses, is similar to eye drug Lucentis and is used in many countries as a low-cost option to treat certain blindness-causing conditions. In its statement, Roche said: “Avastin is not approved for any use in the eye.”
Alam Sher, Punjab’s deputy drug controller told Reuters that counterfeit medicines posed a health risk to patients because their content might be ineffective and contain harmful ingredients.
Sher, who filed the police complaint against the distributors, added that some companies bought Avastin and repackaged it in smaller doses to make it more affordable for patients.
A sharp drop in the value of the local currency against the US dollar has inflated the price of drugs in Pakistan, many of which are either imported or based on imported ingredients. Record high inflation has also diminished the purchasing power of many people.
DRAP banned the distribution of three batches of Avastin, in an order to the Roche Pharma Pakistan, the importer of the Injection, and the Genius Advanced Pharmaceutical Services (GAPS). DRAP officials also raided GAPS, Lahore, and observed unhygienic environment and non-sterile packaging, source said.
The sources said in Islamabad that DRAP banned the sales of Avastin batches numbered H0353B11, B7266B20 and B7266B07. According to sources, a sample of the registered Avastin injection had been sent to the drug testing laboratory.



No comment